 Hot News
Hot News
- 2025 The 22nd QTG Polyurea Technology Training Course (Qtech Training Group, QTG) Enrollment Notice
- Congratulations on the success of the 21st QTG polyurea technology training course
- 2024 The 21st QTG Polyurea Technology Training Course (Qtech Training Group, QTG)
- Academician Sergei Leonovich was invited to visit Qingdao Shamu Advanced Material Co., Ltd.
- In Memoriam of Ding Defu
- Congratulation 60 Years Old of Dudley J. Primeaux
- Pure polyurea is not afraid of testing , just like real gold is not afraid of fire
- The preparation of the second edition of spray polyurea elastomer technology has come to an end
 Experts
Experts

Prof. Weibo HuangGraduated from Sichuan University in 1986 andjoined MCRI (Marine Chemical Research Institute) the same year.

Mr. Dudley J. PrimeauxReceived an M.S. Degree in Organic Chemistry from Lamar University in Beaumont, Texas in 1984.
 Raw material
Raw material
- Qtech-114 Aliphatic Polyurethane Top Coat
- Qtech-112 All Weather Surface Preparation System
- Qtech-404 Mining Equipment Ultra Anti-Abrasion Polyurea Material
- Qtech-400 High Speed Railway Sub Grade Polyurea Protective Coating
- Qtech-406 Elastomer Waterproofing Material
- Qtech-407 Anti-Microbial Polyurea Material
- Qtech-411 Bridge Ultra-Long Service Life Polyurea Material
 Equipments
Equipments
The differences and similarities between polyurethane and polyurea
There are some key differences and similarities between polyurethane and polyurea when the chemistry is understood. A general and simplified reaction of the two systems can be seen in Illustration A.
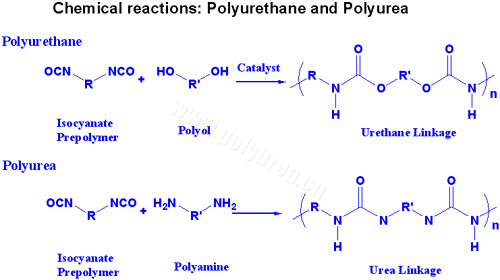
The first thing to notice is that both systems can use the same or similar "A" components. Therefore, the main properties differences are contributed by the "B" component side of the system.
The "B" component of the polyurethane system is comprised of various polyols (polyether, polyester) and normally requires a catalyst in order to cure rapidly. An advantage of the polyurethane system requiring a catalyst is that the catalyst can be adjusted to control the reaction profile to fit the application. Some applications require a smoother finish, and if the system gels too fast, a rough orange peel surface will result.
Furthermore, some applications require the material to flow into the corners or hard-to-spray areas and need a few extra seconds before gelling. This latitude can be accomplished with a polyurethane system choice of catalyst and concentration. The catalyst can be adjusted for a urethane to cure as fast as 10 secs and at low temperatures.
The polyurea system is comprised of polyether-amines or an amine terminated polyol. This polymer is a very reactive polymer and does not require a catalyst (it's an auto-catalytic polymer). This reactivity is typically always fast (in the 5-15 sec range) and cures well on cold surfaces. The reactivity is also so fast and preferential that polyureas in general are moisture insensitive and do not easily react with humidity and moist substrates.
One drawback to the speed of polyureas is that they can be too fast for certain applications that require a smooth surface or a delay time before the system gels.
The Data from "The Polyurea Training Group" of PDA







